REDCap tutorials
This book contains tutorials related to complex functionalities from REDCap as well as a list of new functionalities that are introduced at each major update.
Of note, if in REDCap you are not seeing one of the functionalities presented in the book, it means that you do not have the access rights. To access the desired functionality, go to  , select your username and click on
, select your username and click on  to adjust the individual access privileges. If you are not even seeing the
to adjust the individual access privileges. If you are not even seeing the  option, then you need to ask the project responsible or the Sponsor/PI to grant you access to the desired functionality.
option, then you need to ask the project responsible or the Sponsor/PI to grant you access to the desired functionality.
Recording of the last REDCap Database Implementation Course [10.04.2024]
The course material can be downloaded here.
UniFR Template and Best Practices
UNIFR Template
When requesting the creation of a project to REDCap admins (DIT-SIT, UNIFR), you project will be pre-populated with our own template (courtesy from CTU Bern)
Instruments
In this template we provide example eCRFs (instruments) for standard clinical trials forms:
- Eligibility
- Randomization
- End of Study
- (Serious) Adverse Event (study and sponsor) [specific to IMP trials]
- Protocol Deviation
User roles
| ROLE | DESCRIPTION |
|---|---|
| Analysis | Standard role for the statistician |
| Data Entry | Standard role for the study nurse |
| Monitoring | Standard role for central data monitoring and for on-site monitoring |
| Super User | The project super user has the maximum rights on his study database. He can manage user rights, roles, data access groups (DAGs), he can deploy his study database. |
| Project Setup | Design study database: create eCRFs (instruments), visit plan, reports, enter test data |
| Sponsor/PI | Standard role for the sponsor and/or the PI |
Reports
The template comes with a predefined report that you can use to submit (serious) adverse event cases to the ethics comittee: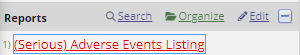
You can adapt the report to your study by going to
 and by clicking on "Edit":
and by clicking on "Edit":
Best Practices for Database Implementation
To improve the quality of your database implementation and make your statistician(s) happy, here are a few tips with courtesy from the CTU Bern.
1. Provide understandable variable names
For each new item, you need to provide a unique variable name. Best practice is to include acronyms in the name of the item corresponding to:
- The instrument in which the item is located
- The label of the item
- The field type
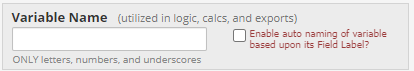
For example, if you want to create an item in a "demographic" instrument assessing whether a participant is a UNIFR student, you could code it accordingly:
dem_unistud_yn
You will find below a list of acronyms that may be used to indicate the field type of an item:
| Field type | Acronym |
|---|---|
| Yes/No | _yn |
| True/False | _tf |
| Date | _dt |
| Date-time | -dtm |
| Integer | _int |
| Numeric | _num |
| Calculated fields | _calc |
| Descriptive text | _desc |
| Notes Box | _txt |
| File upload | _up |
! Be careful that the length of a variable name SHOULD NEVER EXCEED 26 characters !
2. Standardize the coding values
When creating an item and defining choices, try to be consistent across the entire database. You can, for example, use the following coding structure for special values:
| Code | Value |
|---|---|
| 0 | no/false/negative/absent/abnormal |
| 1 | yes/true/positive/present/normal |
| 1 | male |
| 2 | female |
| 88 | other/else |
| 99 | not available/NA/unknown |
Branching Logic Reminder
Here is a small reminder of the most useful fonctions in branching logic and what they can be used for:| Function | Description |
|---|---|
| [sex] = "2" | Display question if sex = female (Female is coded as 2) |
| [sex]= "2" and [given_birth] = "1" | Display question if sex = female (Female is coded as 2) and given birth = yes (Yes is coded as 1) |
| ([height] >= 170 or [weight] < 65) and [sex] = "1" | Display question if (height is greater than or equal to 170 OR weight is lower than 65) AND sex = male (Male is coded as 1) |
| [last_name] <> "" | Display question if last name is not blank (i.e., if last name field has data) |
| [visit_date] = "" | Display question if visit date is blank (i.e., if visit date has no data) |
| [race] = "88" | Display question if race = other (Other coded as 88) |
| [race(88)] = "1" | Display question if 'other race' is checked (Checkbox field type!) |
